
MyndMove™ is a non-invasive Functional Electrical Stimulation (FES) therapy designed to improve voluntary arm and hand function in patients with upper-limb paralysis, such as those suffering from stroke or spinal cord injury (SCI). By stimulating the muscles during specific movements, MyndMove™ helps retrain the brain and body to create new neural pathways, facilitating motor function recovery.
MyndMove™ treatment for lower limb is now commercially available in Canada after MyndTec received Health Canada approval for these indications.
How MyndMove™ Therapy Works
MyndMove™ therapy utilizes Functional Electrical Stimulation (FES) to promote muscle engagement and neuroplasticity. During therapy, electrodes are placed over the muscles, and small bursts of electrical stimulation are delivered as the patient actively attempts movements.
This dual effort, involving both the brain’s signal to the muscle and the stimulation-induced contraction, helps create new neural pathways, leading to improved motor function. Over time, patients gain voluntary control of previously paralyzed muscles, with typical results emerging after 20 hours of therapy. The personalized approach of MyndMove™ adapts to each individual’s progress and specific needs.
MyndMove™ trained physiotherapy or occupational therapy professionals guide patients through tailored, customizable and comfortable protocols that focus on specific movements like grasping, reaching, and pinching. These sessions can result in meaningful motor improvements after 20 hours or more of therapy, with the timing of results varying by individual.
Key Features of MyndMove™
Advanced Neuromodulation Technology
The MyndMove™ system is equipped with advanced embedded algorithm based, customizable stimulation protocols, enabling over 30 different pre-set movement sequences. The system’s 8-channel stimulator allows the stimulation of up to eight muscle groups in a single protocol, facilitating both bilateral arm movement (for SCI patients) and complex unilateral movement (for stroke patients).
Electrical stimulation from the device sends signals to the brain, while patients attempt movements, reinforcing a new motor pathway. Over time, this leads to lasting recovery of voluntary control in paralyzed limbs. It improves voluntary motor control and sensory function in stroke and spinal cord injury (SCI) patients, helping them perform everyday tasks.
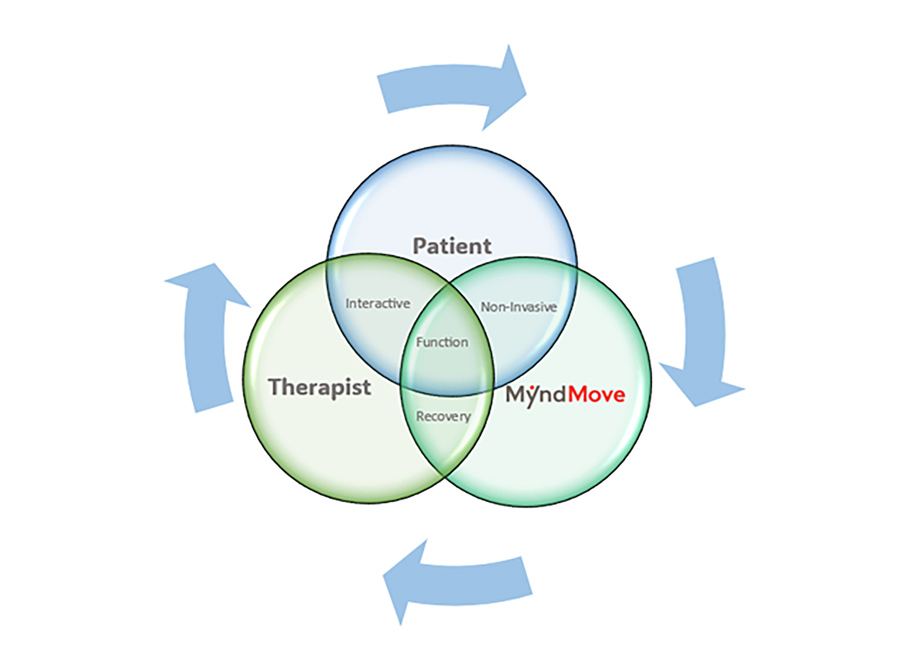
Personalized Interactive Care
The device’s user-friendly and intuitive touchscreen interface allows therapists to tailor therapy sessions based on patient-specific goals, offering seamless customization during treatment. This technology stimulates fine motor control movements
MyndMove’s non-invasive therapy combines patient participation, trained therapists, and a functional electrical stimulation (FES) device to restore voluntary hand and arm function in patients suffering paralysis. MyndMove™ promotes natural movements, offering both bilateral and unilateral stimulation protocols for patients with spinal cord injuries (SCI) and severe hemiparesis.
Neuroplasticity-Driven Recovery
MyndMove FES and NMES therapy supports recovery by not only improving movement but also preventing muscle atrophy, reducing spasms, increasing circulation, and re-educating muscles. MyndMove™ caters to a wide range of patients, from those with hemiplegia post-stroke to individuals with C3-T1 SCI. Please refer to the indications for use in Canada and the United States.
By stimulating neural pathways through both afferent (muscle-to-brain) and efferent (brain-to-muscle) signals, MyndMove™ therapy promotes neuroplasticity. Over time, this helps patients regain lasting voluntary motor control in paralyzed limbs, improving both sensory and motor functions.
Unlike some therapies, MyndMove™ leverages neuroplasticity by retraining the brain’s pathways through targeted electrical stimulation. Over time, patients experience lasting voluntary control of paralyzed limbs, making it a comprehensive long-term solution for improving motor function.
By combining cutting-edge technology, therapist expertise, and patient participation, MyndMove™ offers a powerful tool for restoring motor control in individuals with significant impairments.
MyndMove Indications for Use
Indication for Use in Canada
(licensed for upper limb and lower limb treatment)
MyndMove is an electrical stimulation device indicated for the following uses: Functional Electrical Stimulation (FES)
- Improvement of arm and hand functions and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C3-T1 spinal cord injury.
NeuroMuscular Electrical Stimulation (NMES) for general rehabilitation for:
- Maintenance and/or increase of range of motion.
- Prevention or retardation of disuse atrophy.
- Increase in local blood circulation.
- Reduction of muscle spasm.
- Re-education of muscles.
Indications for Use in the United States
(cleared for upper limb treatment)
MyndMove is an electrical stimulation device indicated for the following uses: Functional Electrical Stimulation (FES)
- Improvement of arm and hand functions and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C3-T1 spinal cord injury.
NeuroMuscular Electrical Stimulation (NMES) for general rehabilitation for:
- Maintenance and/or increase of arm and hand range of motion.
- Prevention or retardation of disuse atrophy.
- Increase in local blood circulation.
- Reduction of muscle spasm.
- Re-education of muscles.
MyndMove™ therapy can only be administered by Trained MyndMove™ Therapists who are physical and occupational therapists and also licensed or certified physical therapist assistants (PTAs) and occupational therapy assistants (OTAs) under their supervision who have completed MyndMove™ training, by MyndTec, on the use of the MyndMove™ system.
Contraindications for Use in USA and Canada
In order for patients to benefit from MyndMove™, they must be medically stable, able to actively participate in and to communicate with the therapist during treatment.
Conditions that preclude patients from receiving MyndMove™ therapy are summarized in our Contraindications for use. These include:
- Do not use the MyndMove™ system if the patient has a pacemaker, implanted defibrillator, or implanted metallic or electronic device. If the patient has passive metallic implants, the therapy can be delivered if the implants are located in an area other than where the electrical stimulation is to be delivered. Patients with pacemakers or an implanted electronic device should not be subjected to stimulation unless specialist medical opinion has first been obtained.
- Do not use the MyndMove™ system if a cancerous lesion is present or suspected on the upper extremity being treated.
- Do not use the MyndMove™ system on an arm if there is an unhealed wound or fracture.
- Do not use the MyndMove™ system over swollen, infected, or inflamed areas or skin eruptions (e.g. phlebitis, thrombophlebitis).
- Do not use the MyndMove™ system if the patient has cognitive impairment. Patient participation is required to deliver therapy and patients must be able to understand and follow instructions.
- Do not use the MyndMove™ system on an arm that has been treated with botulinum toxin in the past 6 months.
For more information on Contraindications for Use and Safety Precautions, please contact us or consult your MyndMove™ User Guide.
Therapist Certification
Trained Therapists Deliver Exceptional Therapy
An integral component of MyndMove™ therapy is the MyndMove™ trained therapist. Physical Therapists (PTs), Occupational Therapists (OTs), licensed or certified Physical Therapist assistants (PTAs) and Occupational Therapy assistants (OTAs) complete an intensive multi-day training course provided by MyndTec Inc. or a partner institute in order to qualify as MyndMove™ Therapists.

Therapists are trained to engage with patients to elicit the desired movements:
- Correctly place electrodes on the skin and optimize muscle stimulation
- Adjust stimulation levels to ensure appropriate muscle contractions
- Ensure the patient is comfortable during therapy
- Identify patient responses to MyndMove™ and ensure patients receive optimal therapy
Therapist Certification
Physical Therapists (PTs), Occupational Therapists (OTs), licensed or certified Physical Therapist assistants (PTAs) and Occupational Therapy assistants (OTAs) undergo a comprehensive course in the fundamental principles of MyndMove™ FES, device operation, protocol selection, and hands-on practice resulting in the therapist being certified for the therapeutic delivery of MyndMove™.
For information on becoming a certified MyndMove™ Therapist, please contact us.
Clinical Evidence
The efficacy of MyndMove™ therapy is supported by numerous clinical studies, including randomized controlled trials in both stroke and spinal cord injury populations.
MyndMove™ is the culmination of years of laboratory and clinical research by Dr. Milos R. Popovic, Toronto Rehab Chair in Spinal Cord Injury Research at the Toronto Rehab Institute – University Health Network, and professor at the Institute of Biomaterials and Biomedical Engineering at the University of Toronto.
Three randomized control studies, two looking at SCI and one looking at stroke patients, examined the effect of FES therapy using the MyndMove™ protocols. These studies found that patients who underwent 40 one-hour long sessions, 3-5 times per week over an 8-12 week period, demonstrated significant and lasting recovery of voluntary arm and hand movement. Patients participating in these studies represented those with some of the most severe deficits in motor function.
Studies have demonstrated the effectiveness of MyndMove™ protocols as FES therapy to improve hand and arm function in:
- Individuals with C3-T1 incomplete SCI
- Individuals with severe hemiplegia due to stroke
Studies evaluated improved hand and arm function based on:
- Self-Care Functional Independence Measure (FIM) score
- Spinal Cord Independence Measure (SCIM) Self-Care Subscore
- Fugl-Meyer Assessment Upper Extremity (FMA-UE) of Motor Recovery after stroke score
Videos highlighting patients participating in this research have been prepared by UHN and the Rehabilitation Engineering Laboratory.
Stroke Rehabilitation
Studies have demonstrated that FES therapy using MyndMove™ protocols can lead to lasting recovery of upper extremity function in individuals who have experienced stroke, including:
- Severe hemiplegia after acute stroke
- Severe chronic hemiplegia due to stroke
Individuals experiencing severe upper extremity paralysis who were 2-7 weeks post-acute stroke took part in the study. 7,8 The patients that underwent one hour sessions of FES therapy with MyndMove™ 5 days a week for 12-16 weeks improved significantly more than patients undergoing conventional occupational therapy for the same timeframe in:
- Object manipulation, palmar grip torque, pinch grip pulling force (P<0.05)
- Barthel Index (P<0.05)
- Self-Care Functional Independence Measure (pictured) (P=0.005)
- Upper extremity Fugl-Meyer scores (P=0.003)
Self-Care Functional Independence Measure (SC-FIM)
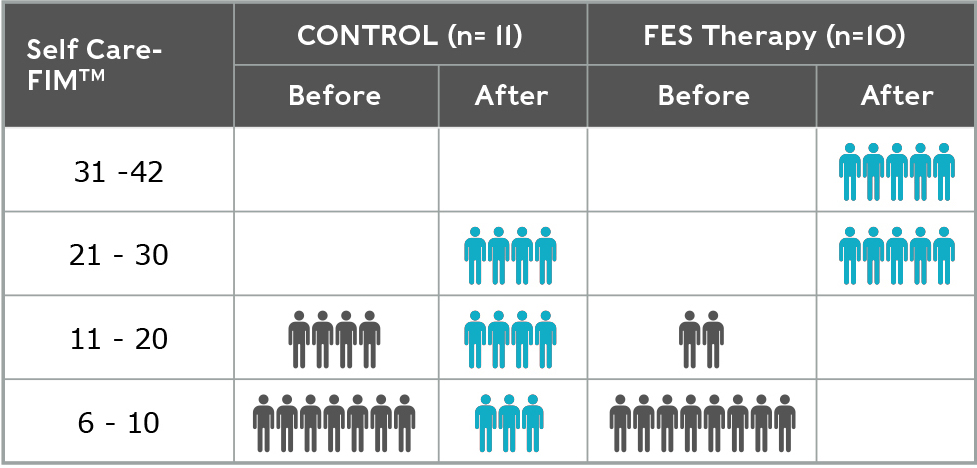
Self-Care Functional Independence Measure (FIM) measures an individual’s ability to feed, bathe, dress, groom, and toilet themselves after stroke. Minimum scores are 6, which indicates full dependence, maximum scores are 42 which indicates full independence.
SCI Rehabilitation
Studies have demonstrated that FES therapy using MyndMove™ protocols can lead to lasting recovery of upper extremity function in individuals with SCI, including:
- Chronic incomplete SCI
- Subacute incomplete SCI
- C3-C7 SCI
Individuals experiencing upper extremity paralysis due to SCI who underwent 40 hours of FES therapy with MyndMove™ + 40 hours of conventional occupational therapy (COT) over an 8 week period improved significantly more than patients who underwent 80 hours of COT during the 8 week period1,5. The patients were treated 5 days per week over the 8 week period. The difference in the mean change in SCIM self-care subscores from baseline to post treatment was statistically significant for the FES + COT group when compared to the COT only group (P< 0.0001).
Spinal Cord Independence Measure (SCIM) Self-Care Subscore
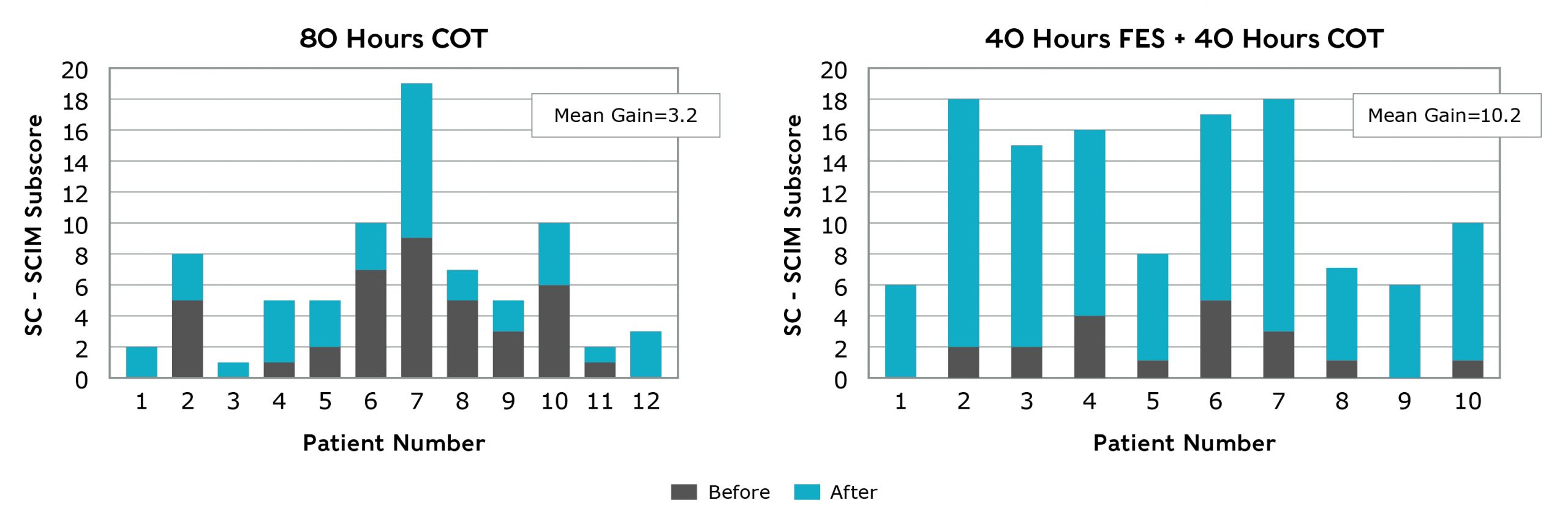
SCIM self-care subscore measures an individual’s ability to feed, bathe, dress, groom and toilet themselves. The minimum score is 0 (dependent) and the maximum score is 20 (independent).
In this study, FES therapy with MyndMove™ delivered by therapists effectively increased independence and thereby improved the quality of life of individuals with tetraplegia when compared with conventional occupational therapy alone.
Ongoing Studies
Active Multicenter Study
Restoration of Reaching and Grasping Function in individuals with Spinal Cord injury using MyndMove™ Neuromodulation Therapy
This study is designed as a multicenter, parallel group, two arm, single-blind, randomized controlled trial to compare the clinical outcomes of MyndMove™ therapy to intensive conventional therapy for individuals with C4-C7 traumatic incomplete SCI with upper extremity paresis. Treatments will commence in the outpatient setting. Study participants will be stratified by rehabilitation site and will be allocated in a 1:1 ratio to the following two treatment arms:
- MyndMove™ therapy: Participants will receive a minimum-maximum of 36-40 one-hour sessions of MyndMove™ therapy for up to 14 weeks.
- Intensive conventional therapy: Participants will receive upper-limb conventional therapy of equivalent frequency, intensity, and duration to MyndMove™ therapy (i.e. a minimum-maximum of 36-40 one-hour sessions of conventional therapy for up to 14 weeks)
- MyndTec Inc.
- U.S. Army Medical Research Acquisition Activity
- United States Department of Defense
- Programs for Assessment of Technology in Health Research Institute
- McMaster University
- Kapadia N, Shaghayegh B, Popovic MR. Influence of Different Rehabilitation Therapy Models on Patient Outcomes: Hand Function Therapy in Individuals with Incomplete SCI. The Journal of Spinal Cord Medicine. 2014:0:0-1.
- Kapadia N, Zivanovic V, Furlan J, Craven BC, McGillivray C, Popovic MR. Functional Electrical Stimulation Therapy for Grasping in Traumatic Incomplete Spinal Cord Injury: Randomized Control Trial. Artif Organs. 2011:35(3):212-216.
- Kapadia N, Zivanovic V, Popovic MR. Restoring Voluntary Grasping Function in Individuals with Incomplete Chronic Spinal Cord Injury: Pilot Study. Topics in Spinal Cord Injury Rehabilitation. 2013:19:4-279.
- Kawashima N, Popvic MR, Zivanovic V. Effect of Intensive FES Therapy on Upper-Limb Motor Recovery After Stroke. Physiotherapy Canada. 2013:65(1):20-28.
- Popovic MR, Kapadia N, Zivanovic V, Furlan J, Craven BC, McGillivray C. Functional Electrical Stimulation Therapy of Voluntary Grasping Versus only Conventional Rehabilitation for Patients with Subacute Incomplete Tetraplegia: A Randomized Clinical Trial. Neurorehabilitation and Neural Repair. 2011:25:5-433.
- Popovic MR, Thrasher T, Adams M, Takes V, Zivanovic V, Tonack M. Functional Electrical Therapy: Retraining Grasping in Spinal Cord Injury. Spinal Cord. 2006:44-143.
- Marquez-Chin C, Bagher S, Zivanovic V, Popovic MR. Functional electrical stimulation therapy for severe hemiplegia: Randomized control trial revisited. Canadian Journal of Occupational Therapy. 2017:84(2):87-97
- Trasher T, Zivanovic V, Mcllroy W, Popovic MR. Rehabilitation of Reaching and Grasping Function in Severe Hemiplegic Patients Using Functional Electrical Stimulation Therapy. Neurorehabilitation and Neural Repair. 2008:22:706-13.
For further details visit NCT03439319
U.S. Department of Defense Clinical Trial Award in SCI: MyndTec is conducting this study through a grant from the U.S. Department of Defense.

